Contents

I last reviewed Abbvie (ABBV) in my November 12, 2022 post at which time I expressed my relief that ABBV was deleveraging. Now, however, ABBV's debt will increase because of its recently announced ImmunoGen (IMGN) acquisition. I am, therefore, not increasing my Abbvie exposure for reasons presented herein.
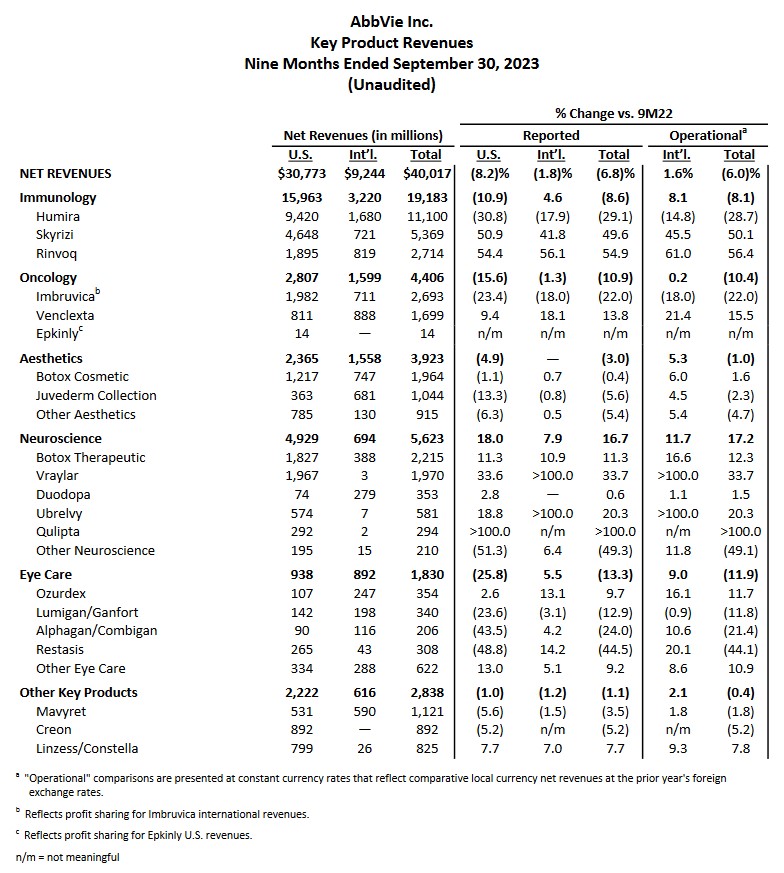
In my previous post, I also commented on the impending expiration of Humira's patent protection in 2023; Humira generated $20.694B (~36.9%) of ABBV's $56.122B FY2021 revenue. It, therefore, comes as no surprise that in the first 3 quarters of FY2023, Humira's total net revenue is only $11.1B (~27.7%) of ABBV's $40.017B YTD2023 total revenue.
ABBV's Skyrizi ($2.939B in FY2021 revenue vs $5.369B in the first 3 quarters of FY2023) and Rinvoq ($1.651B in FY2021 revenue vs $2.714B in the first 3 quarters of FY2023) are immunology drugs that show considerable promise. While ABBV has several other drugs within its pipeline, none are blockbuster drugs like Humira.
In addition, Imbruvica is ABBV's first cancer treatment and is its 3rd largest revenue generator. It, however, is seeing increased competition from similar inhibitors.
Despite a significant drop in ABBV's revenue, it remains profitable and continues to generate strong free cash flow (FCF). In addition,
- Moody's and S&P Global have increased ABBV's domestic unsecured long-term debt credit ratings (see Credit Ratings section below); and
- the dividend metrics are attractive (if dividend metrics happen to be an area of importance for you).
I am not, however, planning to increase my ABBV exposure for the reasons presented below.
ImmunoGen Acquisition
On November 30, ABBV announced a definitive agreement to acquire all outstanding shares of ImmunoGen (IMGN) for $31.26/ in cash.
This transaction values IMGN at a total equity value of ~$10.1B or ~$9.8B net of estimated cash acquired. It is expected to close in mid-2024 and is subject to IMGN shareholder approval, regulatory approvals, and other customary closing conditions.
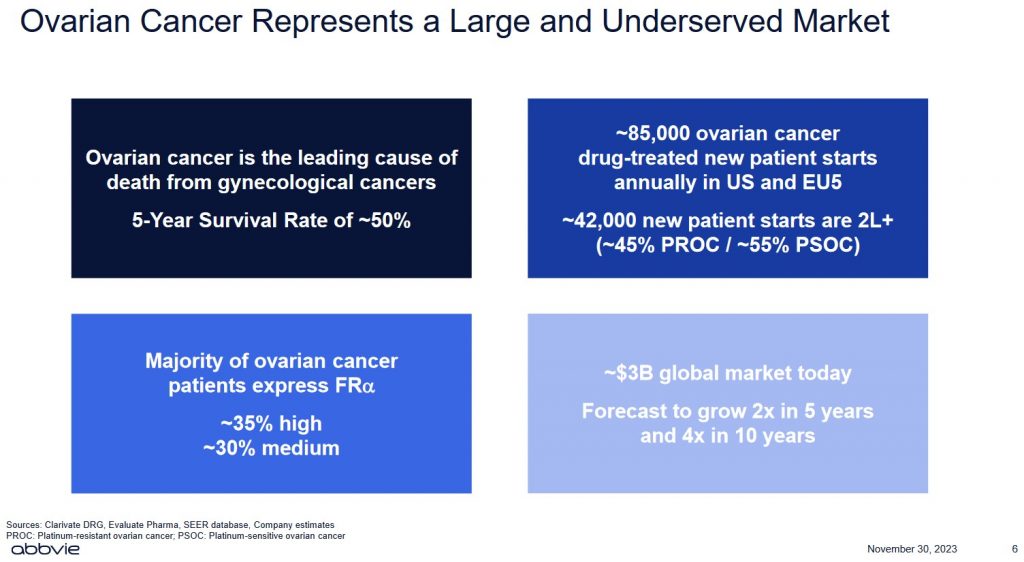
The centrepiece of this impending acquisition is IMGN's Elahere, a first-in-class antibody-drug conjugate (ADC) that the US Food and Drug Administration (FDA) approved in 2022 for ovarian cancer; the FDA is a federal agency of the Department of Health and Human Services.
An ADC is unlike conventional chemotherapy treatments which can damage healthy cells. ADCs are targeted medicines that deliver chemotherapy agents to cancer cells. They deliver the chemotherapy via a linker attached to a monoclonal antibody that binds to a specific target expressed on cancer cells. After binding to the target, the ADC releases a cytotoxic drug into the cancer cell.
Some types of ovarian cancer can move from early stages to more advanced within a year. Others develop more slowly. Experts think that ovarian tumours that begin in the fallopian tubes (as most are thought to) take about 6.5 years to spread to the ovaries.
More than 60% of women live for at least 3 years after being diagnosed with ovarian cancer. Over 50% of patients with ovarian cancer are still alive at least 5 years after diagnosis. Those diagnosed before age 65 do better than older women.
While the global market for ovarian cancer treatment is ~$3B today, forecasts suggest this market could grow to ~$12B in 10 years.
Source: ABBV - Acquisition of ImmunoGen Presentation - November 30, 2023
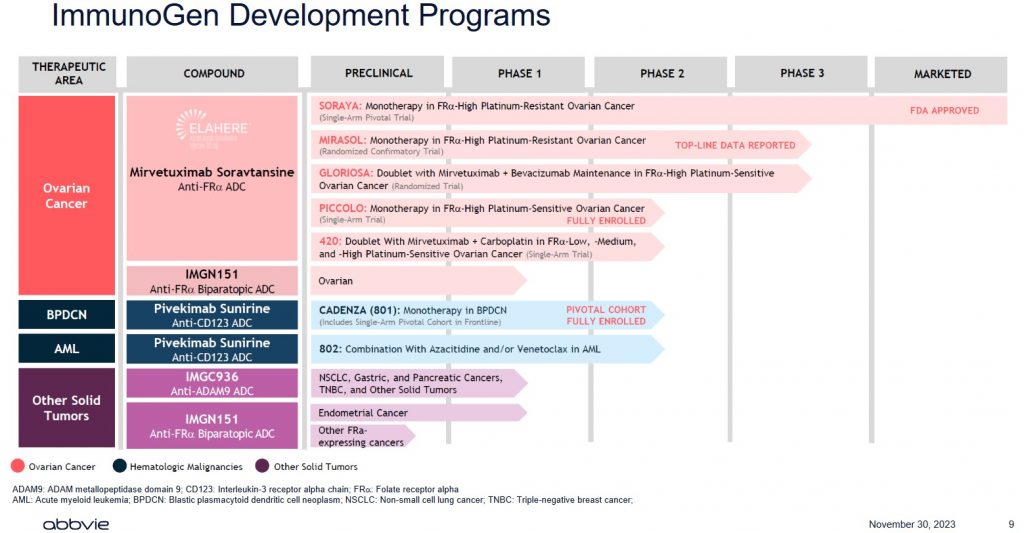
I strongly suspect ABBV has done its homework before making this offer. Investors, however, need to realize that IMGN generates negligible revenue, has never been profitable, has negative free cash flow, and only has 1 drug for which it has received FDA approval.
ABBV is essentially paying up for IMGN's product pipeline. There are no assurances, however, that all the drugs within this pipeline will receive FDA approval!
Source: ABBV - Acquisition of ImmunoGen Presentation - November 30, 2023
The American Cancer Society provides a good explanation of the types and phases of clinical trials.
Developing drugs is extremely time-consuming and costly and most drugs under development never ultimately receive FDA approval. In addition, should a drug receive FDA approval, there is a finite period in which the drug manufacturer's patents are protected. After this timeframe, generic versions of name-brand drugs can be developed; this is exactly what has happened with ABBV's Humira. Furthermore, just because a drug has received FDA approval does not mean that it will be profitable. Another drug company may develop a superior drug using its own formulation. As much as we complain about the cost of medication, it is not surprising why medication can be so expensive.
Furthermore, ABBV's earnings are expected to be negatively impacted in FY2024 and FY2025 and to be neutral in FY2026. Expectations are that this acquisition will only become accretive starting in FY2027. This assumes all goes according to plan.
Financials
Q3 and YTD2023 Results
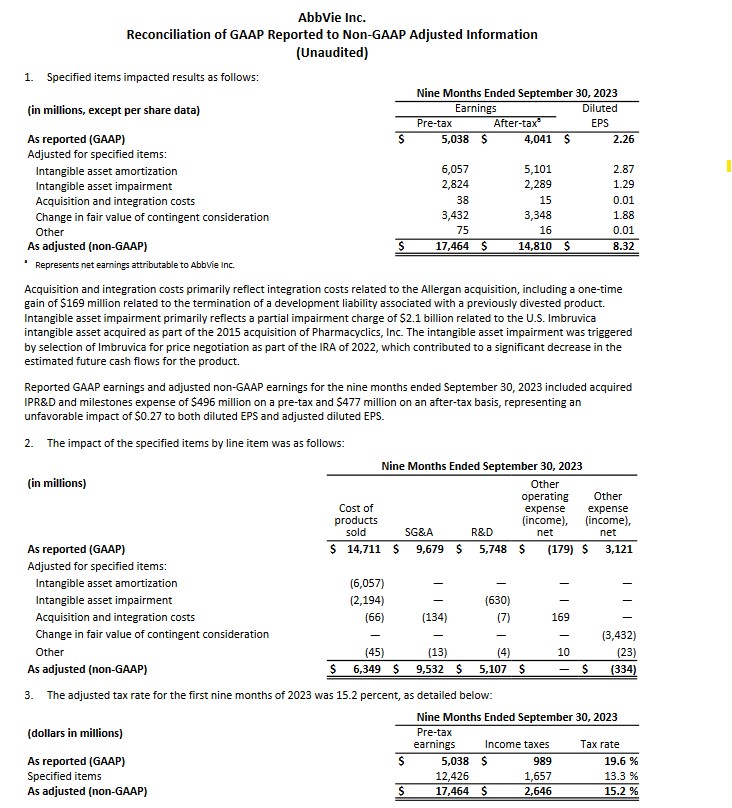
On October 27, ABBV released its Q3 and YTD2023 results and preliminary FY2024 guidance; refer to ABBV's October 27, 2023 Earnings Release and Q3 2023 Form 10-Q. I provide the following for ease of reference in assessing what line items result in the variance between GAAP and non-GAAP earnings.
Source: ABBV - Q3 2023 Form 8-K
Risk Factor - Concentration of Risk
While the risk associated with the loss of Humira's exclusivity in 2023 is evident, investors would be wise to assess ABBV's other risks. A good source of information regarding various Risk Factors is found in Part 1, Item 1A in the FY2022 Form 10-K.
One particular risk investors must consider is that in the US, ABBV distributes pharmaceutical products principally through independent wholesale distributors, with some sales directly to retailers, pharmacies, patients or other customers. In the US, 3 wholesalers (McKesson, Cardinal Health, and Cencora (formerly AmerisourceBerge)) accounted for substantially all of ABBV's pharmaceutical product sales. No individual wholesaler, however, accounted for greater than 39% of ABBV's 2022 gross revenues in the US. Outside the US, ABBV sells products primarily to
wholesalers or through distributors, and depending on the market it works through largely centralized national payers system to agree on reimbursement terms.
Given ABBV's heavy dependence on these 3 wholesalers, it is important to gauge their financial strength.
The credit ratings assigned by S&P Global, Moody's, and Fitch are all investment grade. This gives me a reasonable level of confidence they are unlikely to default on their obligations to ABBV in the foreseeable future.

These were their respective credit ratings at the time of my November 12, 2022 post.
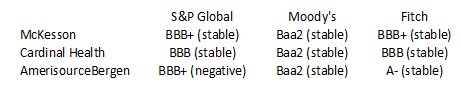
- The A- rating is the bottom tier of the upper medium grade category.
- The BBB+ and Baa1 ratings are the top tier of the lower medium-grade category.
- The BBB and Baa2 ratings are the middle tier of the lower medium grade category.
Outlook
With the release of Q3 and YTD2023 results, ABBV raised its adjusted diluted EPS guidance for FY2023 from $10.86 - $11.06 to $11.19 - $11.23. This includes an unfavourable impact of $0.27/share related to acquired In-Process Research and Development (IPR&D) and milestones expense incurred YTD through Q3 2023. This guidance excludes any impact from acquired IPR&D and milestones that may be incurred beyond Q3, as both cannot be reliably forecasted.
In addition, ABBV raised its FY2024 adjusted diluted EPS guidance floor for FY2024 from $10.70 to $11.00. This also excludes any impact from acquired IPR&D and milestones. This is an update
to ABBV's February 2023 guidance. As a result of this update, ABBV does not expect FY2024 adjusted diluted EPS to be below $11.00/share.
Formal 2024 adjusted diluted EPS guidance is to be released in conjunction with Q4 2023 results.
Long-Term Debt
ABBV's schedule of long-term debt found on page 75 of 162 in the FY2022 Form 10-K will change as cash and debt will be used to finance the IMGN acquisition. Page 19 of 47 in the Q3 2023 Form 10-Q, however, is the most currently available information.
At FYE2022, FYE2021 and FYE2020, ABBV had ~$63.3B, ~$76.7B, and ~$86B of total long-term debt and finance lease obligations with the current portion being ~$4.14B, ~$12.5B, and ~$8.5B and the non-current portion being ~$59.14B, ~$64.2B, and ~$77.6B.
At the end of Q3 2023, the long-term portion was ~$55.6B and the current portion was ~$5.1B.
Credit Ratings
At the time of my prior review, Moody's had assigned a Baa2 domestic senior unsecured long-term debt rating and had upgraded the outlook from stable to positive. In addition, S&P Global had assigned a BBB+ rating with an outlook that was upgraded from stable to positive in November 2022.
On November 17, 2022, Moody's upgraded ABBV from Baa2 to Baa1 and on August 28, 2023, it upgraded ABBV from Baa1 to A3 with a stable outlook.
On November 2, 2023, S&P Global upgraded ABBV from BBB+ to A- with a stable outlook.
Both current ratings are at the bottom tier of the upper medium-grade investment-grade category. These ratinsg define ABBV as having a strong capacity to meet its financial commitments. It is, however, somewhat more susceptible to the adverse effects of changes in circumstances and economic conditions than obligors in higher-rated categories.
On November 30, S&P Global provided the following update:
We believe AbbVie has sufficient capacity at the current rating to cover the cost of this acquisition, notwithstanding the declines in its revenue and EBITDA in 2023. We estimate the purchase will increase its leverage by about 0.4x, though our base-case forecast already assumes about $10 billion of annual acquisition spending in 2024 and 2025 that causes its leverage to rise to 2.4x in 2024 from 2.2x in 2023. More broadly, the rating on AbbVie reflects our expectation its S&P Global Ratings-adjusted leverage will generally remain in the 2x-3x range. Pro forma for this transaction, we estimate the company still has more than $10 billion of debt capacity at the current rating.
While these investment-grade ratings are satisfactory for my purposes, ABBV will have a TON of debt after it acquires IMGN. Investors need to determine if this level of debt meets their risk tolerance.
Dividend and Dividend Yield
We see from ABBV's dividend history that on October 26 it declared a ~4.73% increase in its quarterly dividend ($1.48 increased to $1.55 effective with the dividend payable on February 15, 2024).
With shares currently trading at ~$143.40, the forward dividend yield is ~4.32%.
Since ABBV's inception in 2013, it has increased its quarterly dividend by more than 285%.
It is a member of the S&P Dividend Aristocrats Index, which tracks companies that have annually increased their dividend for at least 25 consecutive years.
Despite ABBV's attractive dividend metrics, investors would be wise not to fixate on them. It is preferable to look at a company's total potential investment return (capital gains and dividend income).
The weighted-average diluted shares outstanding (in millions) in FY2013 - FY2022 are 1604, 1610, 1637, 1631, 1603, 1546, 1484, 1673, 1777, and 1778. For the 3 months that ended September 30, 2023, the weighted average diluted shares outstanding was 1771.
On February 16, 2023, ABBV's Board authorized a $5B increase to the existing stock repurchase authorization. It repurchased 10 million shares for $1.6B during the nine months ended September 30, 2023 versus 8 million shares for $1.1B during the nine months ended September 30, 2022. ABBV's remaining stock repurchase authorization was ~$4.8B as of September 30, 2023.
Valuation
I reference my Valuation comments in the November 12, 2022 post.
ABBV's FY2023 adjusted diluted EPS guidance is $11.19 - $11.23 and its preliminary FY2024 adjusted diluted EPS guidance is expected to be no lower than $11.00/share.
Shares currently trade at $143.40 (December 1 closing price). If we give ABBV the benefit of the doubt that FY2023 results will be $11.23, the forward adjusted diluted PE is ~12.77. Using $11/share for FY2024, we get ~13.
The forward-adjusted diluted PE levels using current broker estimates are:
- FY2023 - 15 brokers - mean of $11.22 and low/high of $10.91 - $11.39. Using the mean estimate I get ~12.8.
- FY2024 - 16 brokers - mean of $11.15 and low/high of $10.70 - $11.83. Using the mean estimate I get ~12.9.
- FY2025 - 15 brokers - mean of $12.19 and low/high of $10.77 - $13.69. Using the mean estimate I get ~11.8.
These might seem like attractive valuations at first blush. If we compare them to the forward-adjusted diluted PE levels for Johnson &Johnson (JNJ) Merck (MRK), Novartis (NVS), Pfizer (PFE), Amgen (AMGN), Sanofi (SNY), Bristol-Myers Squibb (BMY), and Gilead (GILD), and GSK (GSK), ABBV's valuation is reasonably similar. All these companies have their respective challenges and why their valuations appear to be so low is an indication of investor sentiment. We should not, therefore, try to compare their low-teen valuations against companies in other industries that carry far less risk and have superior growth potential.
Final Thoughts
On October 22, 2021, I initiated a 200-share position @ ~$108.70 in a 'Core' account in the FFJ Portfolio. With the automatic reinvestment of the dividend income (after the 15% dividend withholding tax I incur on US holdings held in taxable accounts), I now hold 208 shares.
ABBV needed to do something to replace the loss of revenue and profits because of Humira's loss of exclusivity. Skyrizi and Rinvoq show considerable promise but ABBV needs more blockbuster drugs. It appears ABBV has identified products within IMGN's pipeline that have the potential to generate substantial revenue and profits. I am not, however, a medical expert and have no idea if the potential from IMGN's pipeline justifies the ~$10B purchase price.
Although I am not contemplating exiting my small ABBV position, I certainly have no intention of increasing my exposure other than through the automatic reinvestment of the quarterly dividend income.
Spending ~$10B with the probability of IMGN becoming accretive to earnings in FY2027 (at the earliest) does not fit my risk profile. There are many other companies in which I can invest that have the potential to generate superior returns with much less risk.
As mentioned earlier, ABBV will have a TON of debt after the IMGN acquisition. I would rather invest in existing holdings in the FFJ Portfolio that have no debt or minimal debt.
I wish you much success on your journey to financial freedom!
Note: Thanks for reading this article. Please send any feedback, corrections, or questions to [email protected].
Disclosure: I am long ABBV, JNJ, and MRK.
Disclaimer: I do not know your circumstances and am not providing individualized advice or recommendations. I encourage you not to make any investment decisions without conducting your research and due diligence. You should also consult your financial advisor about your specific situation.
I wrote this article myself and it expresses my own opinions. I am not receiving compensation for it and have no business relationship with any company whose stock is mentioned in this article.