In anticipation of strong Q3 and YTD2021 results, I initiated a 200 shares Abbvie Inc. (ABBV) position @ $108.70/share on October 22, 2021 in one of the ‘Core’ accounts in the FFJ Portfolio.
With the release of Q3 and YTD2021 results on October 29, I now provide this ABBV stock analysis.
ABBV – Stock Analysis – Business Overview
ABBV is a global, research-based biopharmaceutical company that develops and markets advanced therapies that address some of the world’s most complex and serious diseases.
On January 2, 2013, it became an independent biopharmaceutical company as a result of the distribution by Abbott Laboratories (ABT) of 100% of the outstanding common stock of ABBV to ABT’s shareholders.
On May 8, 2020, ABBV completed the acquisition of Allergan plc for ~$63B.
Following the closing of the Allergan acquisition, ABBV implemented an integration plan designed to reduce costs, integrate and optimize the combined organization. The integration plan is expected to realize more than $2B of expected annual cost synergies over 3 years, with approximately 50% realized in R&D, 40% in selling, general and administrative (SG&A) and 10% in the cost of products sold. To achieve these integration objectives, ABBV is expected to incur ~$2B of charges through 2022. These costs will consist of severance and employee benefit costs (cash severance, non-cash severance, including accelerated equity award compensation expense, retention and other termination benefits) and other integration expenses.
The company’s product portfolio and pipelines (see here and here) reveal a comprehensive product portfolio with leadership positions in key therapeutic areas of immunology, hematologic oncology, aesthetics, neuroscience, eye care and women’s health.
Its therapeutic focus areas are:
- Immunology
- Oncology
- Neuroscience
- Virology
- Eye Care
In the United States, ABBV distributes pharmaceutical products principally through independent wholesale distributors, with some sales directly to retailers, pharmacies and patients.
In 2020, three wholesale distributors (McKesson Corporation, Cardinal Health, Inc. and AmerisourceBergen Corporation) accounted for substantially all of ABBV’s sales in the US. No individual wholesaler accounted for more than 38% of ABBV’s 2020 gross US revenues.
Outside the US, ABBV sells products primarily to customers or through distributors, depending on the market served.
In addition, certain products are co-marketed or co-promoted with other companies. ABBV has no single customer that, if the customer were lost, would have a material adverse effect on the company’s business.
Humira – Loss of Exclusivity
Humira, one of ABBV’s immunology products, is the company’s single largest product. It accounted for just over 37% of ABBV’s total net revenues for the 9 months ended September 30, 2021.
This product is sold in numerous markets and is used to treat multiple conditions for which a list is found on page 5 of 155 in the FY2020 10-K.
According to ABBV, the global immunology market represents an $80B value with more than 25,000,000 patients treated.
2023 will mark the official end of ABBV’s exclusivity over the highest-grossing drug in the world. It has entered into settlements with nine manufacturers to allow for a 2023 launch of biosimilars.
Biosimilars are not new drugs, but rather are copies of biologic drugs that have been used to treat many diseases and conditions. They differ from generic drugs mainly because while generic drugs are identical to the original in chemical composition, biosimilar drugs are close enough in duplication to accomplish the same therapeutic and clinical result. Another key difference is that generics are copies of synthetic drugs, while biosimilars are modelled after drugs that use living organisms as important ingredients.
Although ABBV will lose exclusivity in 2023, some manufacturing patents extend to 2034. Other biosimilar manufacturers will, therefore, need to pursue individual patent procedures with ABBV to launch their own candidates.
Skyrizi and Rinvoq
With the approaching pressures of loss of exclusivity of its largest single product, ABBV has been developing Skyrizi and Rinvoq. These two resilient next-generation immunology drugs already represent ~20.7% of Humira sales and are expected to increase to a third of Humira sales in 2022.
Significant Programs and Developments
At the Morgan Stanley Virtual 19th Annual Global Healthcare Conference in mid-September, ABBV’s Chairman/CEO stated the company expects excellent long-term growth from both resilient next-generation immunology drugs. The FDA recently announced the oral surveillance data and the company is working with the agency on labelling.
In addition, the company’s broad portfolio of growth assets gives management confidence that after Humira’s loss of exclusivity the company should rapidly return to growth in 2024 and maintain high single-digit growth through the end of the decade.
Item 2 – Management’s Discussion and Analysis of Financial Condition and Results of Operations in the Q2 10-Q includes sections that summarize transitions of significant programs from mid-stage development to late-stage development as well as developments in significant late-stage and registration programs; ABBV expects multiple mid-stage programs to transition into late-stage programs in the next 12 months.
The Q3 10-Q is not yet available but the Recent Events segment in the Q3 Form 8-K provides an update on ABBV’s pipeline.
Part 1 of ABBV’s FY2020 10-K has a comprehensive overview of the company’s products, marketing, sales and distribution capabilities, competitive landscape, the regulatory environment, risk factors, and more.
ABBV – Stock Analysis – Financials
Q3 and YTD2021 Results
Form 8-K (link provided above) reflects ABBV’s Q3 and YTD2021 results.
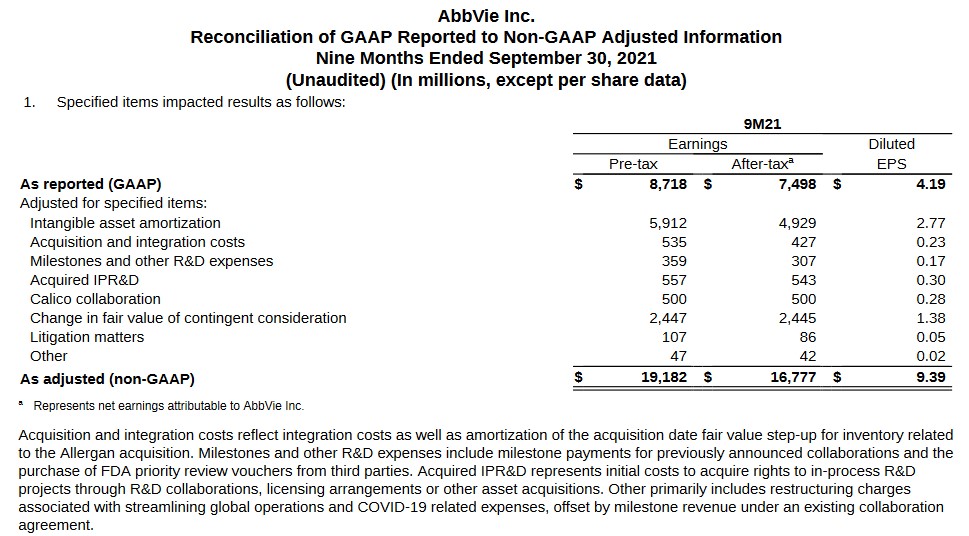
The following reconciliation discloses the various adjustments made to arrive at non-GAAP earnings. We can likely expect ongoing sizable intangible asset amortization adjustments as this relates to the $63B Allergan plc acquisition; this is a non-cash charge.
The promising Skyrizi and Rinvoq drugs currently generate only ~0.8% of ABBV’s YTD Net Revenue. However, Skyrizi’s global sales of ~$0.8B were up 18.1% reflecting continued market share gains. In the U.S., Skyrizi’s leading psoriasis patient share, which includes both new and switching patients, is now ~36%. This is more than double the share capture of the next nearest biologic competitor.
Biologics fight the causes rather than just ease the symptoms. They target a specific part of the immune system and block certain cells or proteins that play a role in psoriasis. While this helps with inflammation and other issues, it also lowers our body’s defenses. If we have a weaker immune system we could be more vulnerable to infections or diseases. It is, therefore, important to watch for signs of an infection (eg. fever, chills, and feeling tired or sore).
Skyrizi’s total prescription share in the U.S. psoriasis biologic market is now ~20%, second only to Humira. Internationally, Skyrizi continues to grow, having achieved patient share leadership in more than a dozen key markets.
In Q3, Skyrizi sales jumped 18% to $0.796B. In the U.S. psoriasis market, this drug is leading all biologics with a 36% share among new and switching patients.
This compelling performance is to be further supported by two important near-term enhancements.
- ABBV has received a positive response to the recent launch of its new and convenient Skyrizi single-dose 150-milligram self-injectable pen and syringe in major territories around the world. Skyrizi is currently the only quarterly dose brand that is available in a single self-injectable pen for patients.
- Preparation is underway for the global launch of Skyrizi in psoriatic arthritis as ABBV nears approval decisions in both the U.S. and Europe. It received a CHMP positive opinion in early October with anticipated approval in Europe by year-end and FDA approval anticipated early 2022.
CHMP is the Committee for Medicinal Products for Human Use and is the European Medicines Agency’s committee responsible for human medicines.
Once approval is received it will round out Skyrizi’s dermatology label and give patients with psoriatic arthritis access to a new compelling therapeutic option. ABBV is also making excellent progress with Skyrizi’s development in Crohn’s disease. This was recently submitted for U.S. regulatory review with commercialization expected in 2022.
Rinvoq’s global sales of $0.453B were up nearly 20% from Q2 2021. It now has a ~17% patient market share in the U.S. as well as leadership in half a dozen key countries.
Rinvoq’s current label covers rheumatoid arthritis and ABBV is awaiting The Food and Drug Administration’s (FDA) decisions on atopic dermatitis, psoriatic arthritis and ankylosing spondylitis. It has also recently filed Rinvoq for ulcerative colitis where the drug’s clinical data have outperformed what ABBV had originally expected in drafting up its 2025 sales guidance.
Recently, the FDA informed the following 3 pharmaceutical companies of the need for more stringent heart safety and cancer warnings for:
- Rinvoq (ABBV)
- Xeljanz (Pfizer)
- Olumiant (Lilly)
All three drugs belong to the same Janus kinase (JAK) inhibitors class of medicines and they currently already bear warnings about blood clots and lymphoma; these inhibitors help ease joint pain and swelling.
Since the final FDA label for Rinvoq remains forthcoming, ABBV is not ready to reconfirm its guidance which calls for Rinvoq’s risk-adjusted 2025 sales at $8B as of December 2020. ABBV’s CEO isn’t sure how much damage the update will do but remains confident this asset will be a major contributor to ABBV’s long-term growth.
FY2021 Outlook
ABBV has just raised FY2021 GAAP diluted EPS guidance from $6.04 – $6.14 to $6.29 – $6.33 and raising FY2021 adjusted diluted EPS from $12.52 – $12.62 to $12.63 – $12.67. The adjusted guidance excludes $6.34/share of intangible asset amortization expense, non-cash charges for contingent consideration adjustments and other specified items.
Q4 net revenue is expected to be ~$15B.
Free Cash Flow (FCF)
ABBV’s strong performance continues to support capital allocation priorities. YTD, ABBV has generated ~$17B of FCF and its cash balance at the end of Q3 was ~$12B.
Long-Term Debt
The most current schedule of long-term debt is found on page 89 of 268 in the FY2020 10-K. At FYE2020, ABBV had ~$86B of total long-term debt and finance lease obligations with the current portion being ~$8.5B and the non-current portion being ~$77.6B.
At the end of Q2 2021, the current portion was ~$7.9B and the long-term portion was ~$74.2B (page 5 of 45 in the Q2 10-Q).
The Q3 10-Q is not yet available but management has indicated the company remains on track to achieve ~$17B of cumulative debt paydown by the end of FY2021 and further deleveraging through 2023. Management anticipates ABBV’s net leverage ratio to be reduced to 2.3 times by FYE2021 and ~2 times by FYE2022.
NOTE: ABBV’s net leverage ratio means, with respect to any Test Period, the ratio of (a) Consolidated Total Net Indebtedness as of the last day of such Test Period to (b) Consolidated EBITDA for such Test Period.
ABBV – Stock Analysis – Credit Ratings
ABBV’s senior unsecured long-term debt is currently rated:
- Moody’s: Baa2 with a stable outlook
- S&P Global: BBB+ with a stable outlook
The rating assigned by S&P Global is the top tier of the lower-medium grade investment-grade category and is one tier above Moody’s.
Both ratings define ABBV as having an ADEQUATE capacity to meet its financial commitments. However, adverse economic conditions or changing circumstances are more likely to lead to a weakened capacity of the obligor to meet its financial commitments.
ABBV – Stock Analysis
Dividend and Dividend Yield
ABBV’s dividend history is accessible here.
On October 29, 2021, ABBV declared a ~8.5% increase in the company’s quarterly cash dividend from $1.30/share to $1.41/share beginning with the dividend payable on February 15, 2022 to shareholders of record as of January 14, 2022.
Since the company’s inception in 2013, it has increased its quarterly dividend by more than 250%.
The weighted-average diluted shares outstanding (in millions) in FY2011 – FY2020 is 1577, 1577, 1604, 1610, 1637, 1631, 1603, 1546, 1484, and 1673.
ABBV – Stock Analysis – Valuation
ABBV has generated YTD diluted EPS of $4.19 and FY2021 guidance is now $6.29 – $6.33. Using the $6.31 mid-point and the current ~$114.70 share price, the forward diluted PE is ~18.
FY2013 – FY2020 PE levels are 18.51, 28.45, 34.64, 16.92, 23.47, 19.13, 39.00, and 23.09. There is little point, however, in comparing ABBV’s current valuation with historical levels because the current version of ABBV is nothing like that before the May 8, 2020 Allergan plc acquisition.
YTD adjusted diluted EPS is $9.39 and FY2021 guidance is $12.63 – $12.67. Using the $12.65 mid-point, the forward adjusted diluted PE is ~9.
Using the current ~$114.70 share price and broker guidance derived from the two online trading platforms I use, the forward adjusted diluted PE is:
- FY2021 – 19 brokers – mean of $12.60 and low/high of $12.17 – $12.75. Using the mean estimate I get ~9.
- FY2022 – 17 brokers – mean of $13.92 and low/high of $12.87 – $14.70. Using the mean estimate I get ~8.2.
- FY2023 – 14 brokers – mean of $12.17 and low/high of $11.53 – $13.40. Using the mean estimate I get ~9.4.
Investing in ABBV is not without risk. Investors should consider the drop in brokers’ guidance for FY2023 before investing in ABBV.
ABBV – Stock Analysis – Final Thoughts
I anticipate ABBV will continue to trade at a low valuation multiple because other biosimilars will very likely launch against immunology drug Humira in 2023. However, ABBV is making good progress in developing drugs that will generate revenue and profitability to somewhat offset Humira’s impending loss of exclusivity. While these drugs are under development I expect ABBV will continue to generate strong FCF we can expect to see a significant debt reduction.
Despite a slight uptick in ABBV’s share price after my purchase, shares are attractively valued and offer a margin of safety to compensate for the risks.
I wish you much success on your journey to financial freedom!
Note: Thanks for reading this article. Please send any feedback, corrections, or questions to finfreejourney@gmail.com.
Disclosure: I am long ABBV.
Disclaimer: I do not know your circumstances and am not providing individualized advice or recommendations. I encourage you not to make any investment decisions without conducting your research and due diligence. You should also consult your financial advisor about your specific situation.
I wrote this article myself and it expresses my own opinions. I am not receiving compensation for it and have no business relationship with any company whose stock is mentioned in this article.