The phrase ‘exit stage right’ is a playwright’s way of giving clear directions for a character to leave the stage. This is exactly what I have done with my Abbvie (ABBV) exposure.
In my December 2 Why I Am Not Increasing My AbbVie Exposure post, I explain my decision not to add to my ABBV exposure following the announcement to acquire all outstanding shares of ImmunoGen (IMGN) for $31.26/ in cash; this transaction is a premium of ~95% of IMGN’s closing share price on November 30.
At this purchase price, ABBV has assigned a total equity value of ~$10.1B or ~$9.8B net of estimated cash acquired.
A mid-2004 closing forecast is subject to IMGN shareholder approval, regulatory approvals, and other customary closing conditions.
My concerns with this acquisition are that ABBV’s:
- debt level will increase significantly. However, Moody’s and S&P Global are not amending ABBV’s credit ratings;
- earnings are expected to be negatively impacted in FY2024 and FY2025 and to be neutral in FY2026. Expectations are that this acquisition will only become accretive starting in FY2027. This assumes all goes according to plan.
Cerevel Acquisition
Following the December 6 market close, ABBV announced a definitive agreement to acquire Cerevel Therapeutics (CERE) and its neuroscience pipeline of multiple clinical-stage and preclinical candidates with potential across several diseases including schizophrenia, Parkinson’s disease (PD), and mood disorders. This acquisition complements ABBV’s neuroscience portfolio, adding a wide range of potentially best-in-class assets that may transform standards of care across psychiatric and neurological disorders where significant unmet needs remain for patients.
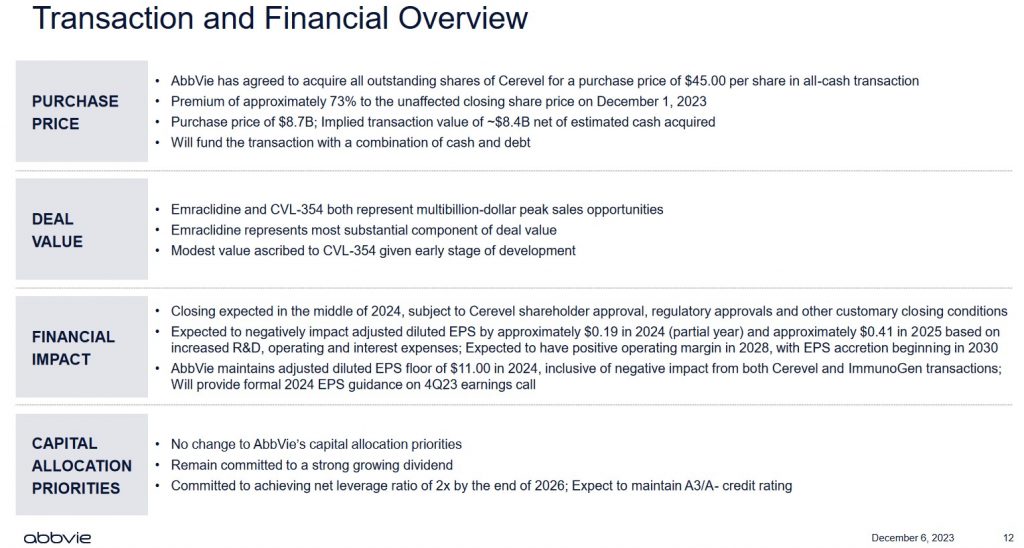
This transaction values CERE at $45.00/share for a total equity value of ~$8.7B; the implied transaction value is ~$8.4B net of estimated cash acquired.
A mid-2024 closing is forecast but is subject to CERE shareholder approval, regulatory approvals, and other customary closing conditions.
The US Food and Drug Administration website an explanation of the various phases in the Drug Development Process. A drug in phase 3 means human testing has been conducted to make sure it is safe and effective. Having a drug in this phase, however, is no assurance it will ultimately receive FDA approval.
Furthermore, there is always a chance a competitor’s drug could be superior if the drug does ultimately make it to market.
Looking at CERE’s pipeline, it currently has only 1 drug in phase 3.
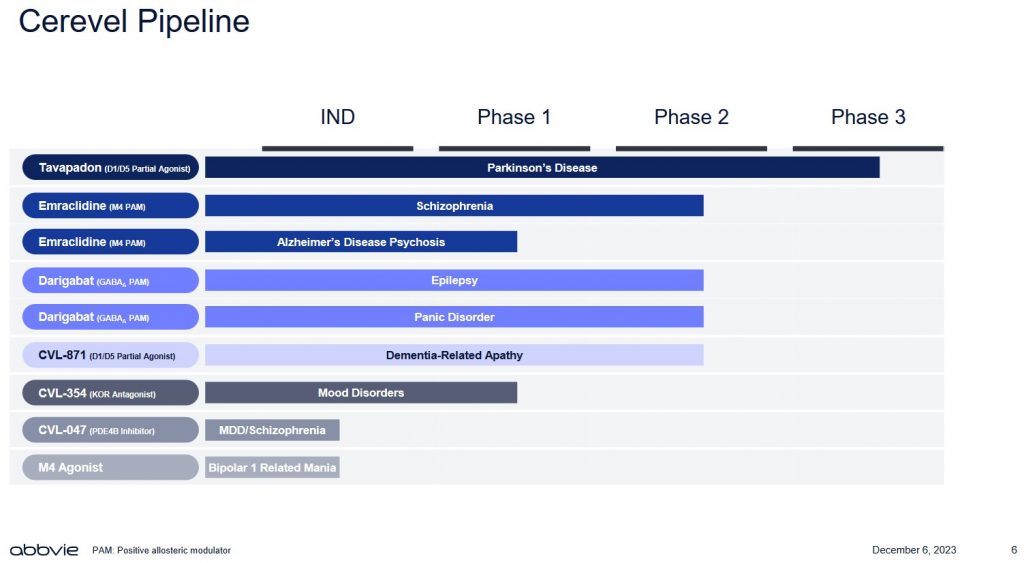
Source: ABBV – Acquisition of Cerevel Presentation – December 6, 2023
ABBV is paying ~$8.7B for ‘potential’ as CERE has no income producing drugs; page 7 of 93 in CERE’s Q3 2023 Form 10-Q discloses $0 revenue!
The CERE acquisition might make strategic sense. This, however, is the second acquisition announcement within days where EPS accretion is not expected for several years; EPS accretion is forecast to begin in 2030.

Source: ABBV – Acquisition of Cerevel Presentation – December 6, 2023
Final Thoughts
I have absolutely no idea if the IMGN and CERE pipelines will lead to blockbuster drugs to replace the lost revenue resulting from ABBV’s Humira patent expiry. Even if they end up receiving FDA approval, it will be several years before these acquisitions become accretive to ABBV’s earnings.
ABBV expects no change to its A3 (Moody’s) and A- (S&P Global) ratings. Its debt load, however, is going to be well beyond my risk tolerance.
At the end of Q3 2023, ABBV had ~$124B of total debt. Total current liabilities were ~$34.8B and total current assets were ~$33.2B of which ~$13.3B was cash and equivalents.
ABBV states it will fund these transactions with a combination of cash and debt. A sizable portion of the cash on hand, however, is to service short-term liabilities. It is, therefore, likely ABBV will raise debt to fund the majority of the ~$18.2B to close these two acquisitions. I envision ABBV’s total debt swelling to at least $135B once the acquisitions close.
Fortunately, ABBV generates strong Free Cash Flow (FCF) because neither acquisition is likely to contribute to ABBV’s FCF over the next few years.
I am also concerned that the Biden administration has asserted its authority to seize the patents of certain costly medications in an effort to slash high drug prices and promote more competition in the US pharmaceutical industry. Depending on the outcome of the next Presidential election, this could change. I am, however, concerned that the rules of engagement could change.
Patent terms are set by statute. Currently, the term of a new patent is 20 years from the date on which the application for the patent was filed in the US. Many other factors, however, can affect the duration of a patent.
Suppose a pharmaceutical company develops a drug with expectation that its patent will be good for 20 years. Several years later, however, the US federal government decides to reduce this time period. This certainly blows a hole through profitability estimates made at the time of development!
Perhaps the recent acquisitions will pan out. ABBV, however, no longer meets my low tolerance for risk. Given this, I deem it prudent to exit stage right.
On October 21, 2021, I initiated an 200 share position in a ‘Core’ account in the FFJ Portfolio at an average cost of ~$108.70. With the automatic reinvestment of the quarterly dividends, I ended up with 208 shares with an average cost of ~$110.26.
On December 7, I sold all 208 shares @ $146.53/share for a ~$36.27/share profit.
I wish you much success on your journey to financial freedom!
Note: Thanks for reading this article. Please send any feedback, corrections, or questions to finfreejourney@gmail.com.
Disclosure: I no longer have exposure to ABBV.
Disclaimer: I do not know your circumstances and am not providing individualized advice or recommendations. I encourage you not to make any investment decisions without conducting your research and due diligence. You should also consult your financial advisor about your specific situation.
I wrote this article myself and it expresses my own opinions. I am not receiving compensation for it and have no business relationship with any company whose stock is mentioned in this article.