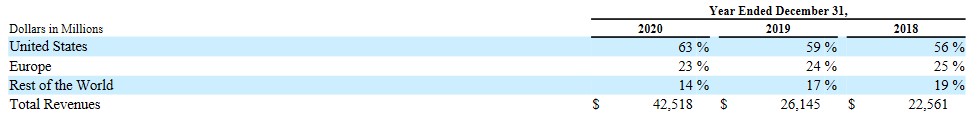The irrational exuberance we are currently witnessing has led to a significant reduction in the number of attractively valued high-quality companies. Despite this challenge, however, there are some companies with the potential to generate an attractive total return. This Bristol-Myers Squibb stock analysis explains why BMY is one such company. Despite its attractiveness, I do not intend to initiate a position.
Information Sources
The following are links to some of my sources to aid in your own analysis.
Part 1 of the FY2020 10-K has a good overview of BMY’s major businesses, customers, business strategy, solutions and risks. Additional information is also available in the:
Bristol-Myers Squibb – Stock Analysis – Industry Overview
BMY owns or licenses several patents in the U.S. and foreign countries primarily covering its products. It has also developed many brand names and trademarks for its products.
The overall protection of patents, trademarks, licenses and other intellectual property rights are of material value and act to protect these rights from infringement.
In the pharmaceutical industry, the majority of an innovative product’s commercial value is usually realized during the period in which the product has market exclusivity. A product’s market exclusivity is generally determined by two forms of intellectual property: patent rights held by the innovator company and any regulatory forms of exclusivity to which the innovative drug is entitled.
Regulatory Data Protection (RDP) exclusivity rights sometimes influence market exclusivity. This is an intellectual property right available for a limited duration which protects an innovator’s proprietary safety and efficacy data for its innovative product. It prevents any other party, during the RDP term, from relying on the innovator’s proprietary data to obtain marketing authorizations and market follow-on generic products
- Competition;
- Pricing, Price Constraints and Market Access;
- Government Regulation;
- Sources and Availability of Raw Materials;
- Manufacturing and Quality;
- Environmental Regulation;
- Human Capital Management and Resources; and
- Foreign Operations.
Bristol-Myers Squibb – Stock Analysis – Business Overview
BMY operates in one segment (Biopharmaceuticals) and is engaged in the discovery, development, licensing, manufacturing, marketing, distribution and sale of biopharmaceutical products on a global basis. The company focuses is on helping to address the unmet medical needs of patients with serious diseases. In FY2020, BMY invested $9.2B in R&D, which included the discovery and development of new medicines. A list of its marketed products is accessible here.
It is one of the four largest U.S.-based biopharmaceutical companies following the ~$80.3B acquisition of Celgene (completed November 20, 2019) and the ~$13.1B acquisition of MyoKardia (completed November 17, 2020).
The percentage of revenues by significant region/country were as follows:
The significant increase in BMY’s Total Revenue is attributed to the Celgene megamerger.
BMY faces challenging patent losses and is aggressively repositioning itself. It has shed its diabetes business, medical imaging group, wound care division, and nutritional business to focus on the high-margin specialty drugs.
The acquisition of Celgene has moved BMY further into the specialty pharmaceutical segment of the market. Celgene’s drugs largely target cancer and this is an area that tends to have strong drug pricing power.
The Celgene acquisition strengthened BMY’s late-stage pipeline which, before the Celgene acquisition, was sparse. BMY now has products that aim to treat multiple sclerosis, inflammatory bowel disease, anemia and cancer; three of these products, Inrebic (cancer) Reblozyl (anemia) and Zeposia (multiple sclerosis) received FDA approval during the past 3 years.
If BMY can continue to advance late-stage pipeline projects through approval and successful commercialization, this should reduce its need to pursue large, strategic acquisitions in the intermediate-term.
In addition to developing products on its own, BMY has a long history of successful scientific and commercial collaborations. External innovation and partnering have brought significant commercial success and pipeline growth with 12 of BMY’s 20 blockbuster medicines having been derived from collaborations. In addition, more than 60% of BMY’s current development pipeline is externally sourced.
All of the above should help BMY maintain its drug pricing ability during a period in which governments and private payers are pushing back on drug prices.
$6.4B Lawsuit Over Delayed Cancer Drug
Large pharmaceutical companies are continually involved in various lawsuits, claims, government investigations and other legal proceedings that arise in the ordinary course of business. These claims or proceedings can involve various types of parties, including governments, competitors, customers, suppliers, service providers, licensees, employees, or shareholders, among others. These matters may involve patent infringement, antitrust, securities, pricing, sales and marketing practices, environmental, commercial, contractual rights, licensing obligations, health and safety matters, consumer fraud, employment matters, product liability and insurance coverage, among others. The resolution of these matters often develops over a long period of time and expectations can change as a result of new findings, rulings, appeals or settlement arrangements.
BMY’s Q1 2021 10-Q has over 5 pages of different active legal proceedings (Note 17 commencing on page 20 of 55).
After the release of Q1’s 10-Q, BMY was sued in early June 2021 for $6.4B for allegedly delaying its Breyanzi cancer drug to avoid payments to shareholders of the former Celgene. The complaint filed in Manhattan federal court accuses BMY of failing to use contractually required ‘diligent efforts’ to win U.S. Food and Drug Administration approval for the non-Hodgkin lymphoma drug by a December 31, 2020 deadline; it won FDA approval for Breyanzi on February 5, 2021. By missing this deadline, BMY is excused from owing an additional $9/share in cash to Celgene shareholders; this article provides additional details.
Legal matters are typically very costly and protracted affairs and the outcome is typically uncertain. The magnitude of the lawsuit is significant and investors need to factor into the investment decision-making process the possibility of BMY being unsuccessful in its defence.
Bristol-Myers Squibb – Stock Analysis – Financials
Q1 2021 Results and FY2021 Guidance
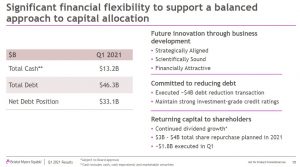
In Q1, BMY’s balance sheet strength and strong cashflow generation enabled it to accelerate ~$4B in debt repayment and share repurchases of ~$1.8B. BMY’s acceleration in debt repayment demonstrates its strong commitment to its investment-grade credit rating. The plan is to also continue to return cash to shareholders through dividends and share repurchases.
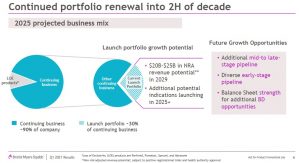
BMY’s focus is on growing the business between 2020 and 2025. Expectations are that in 2025, BMY’s ‘Loss of Exclusivity’ products will constitute less than 10% of the business with ~33% of continuing business coming from the company’s launch portfolio. Management believes the new launch portfolio has significant potential, with $20B – $25B of non-risk-adjusted sales potential in 2029; this excludes the potential medicines that could come from BMY’s mid- or early-stage pipeline.
BMY’s liquidity position remains strong with ~$13B in cash and marketable securities. In addition, it generated, more than $3.8B of Operating Cash Flow and ~$3.65B of Free Cash Flow in Q1.
On the capital allocation front, business development remains BMY’s top priority and opportunities will continue to be evaluated to complement internal innovation.
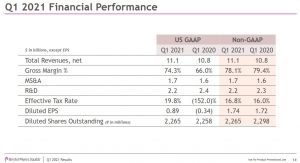
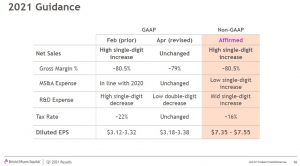
BMY’s 2021 GAAP EPS guidance range is now $3.18 – $3.38 versus the prior $3.12 – $3.32. The non-GAAP EPS guidance range of $7.35 – $7.55 remains unchanged.
Bristol-Myers Squibb – Stock Analysis – Credit Ratings
My risk tolerance is low, and therefore, I invest primarily in companies whose credit ratings are investment grade.
In early 2002, Moody’s rated BMY’s senior unsecured domestic debt AAA. This rating was downgraded 5 notches by September 2006 to its current A2 level. This rating is the middle tier of the upper-medium grade category.
S&P Global currently assigns an A+ rating to BMY’s senior unsecured domestic debt with a negative outlook. This rating is the top tier of the upper-medium grade category.
Fitch assigned an A- rating (the bottom tier of the upper-medium grade category) commencing July 2013 but withdrew its ratings for commercial reasons on June 7, 2021.
The ratings are investment grade and define BMY as having a STRONG capacity to meet its financial commitments. However, BMY is somewhat more susceptible to the adverse effects of changes in circumstances and economic conditions than obligors in higher-rated categories.
Merck & Co., Inc.’s (MRK) unsecured domestic long-term debt, on the other hand, is rated A1, A+, and A+ by Moody’s, S&P Global and Fitch. In addition, Johnson & Johnson’s (JNJ) unsecured domestic long-term debt is rated Aaa and AAA by Moody’s and S&P Global. Fitch withdrew its AAA rating in July 2019 for commercial reasons.
All ratings are acceptable from my perspective but I prefer MRK and JNJ.
Bristol-Myers Squibb – Stock Analysis – Dividends and Share Repurchases
Dividend and Dividend Yield
Although BMY has a lengthy history of dividend distributions, we see several historical dividend increases have been relatively insignificant.
The current $0.49/share/quarter dividend ($1.96/year) provides investors with a ~2.95% dividend yield based on the current $66.50 share price. If you are a Canadian taxpayer like me and hold BMY shares in a taxable account, you incur a 15% withholding tax. Your annual dividend is ~$1.67/share and your dividend yield is ~2.5%.
By way of comparison, MRK’s $0.65/quarterly dividend yields ~3.33% based on MRK’s current ~$78 share price or ~2.83% if you are a Canadian citizen and hold shares in a taxable account.
JNJ’s $1.06/quarterly dividend yields ~2.55% based on JNJ’s current ~$166 share price or ~2.17% if you are a Canadian citizen and hold shares in a taxable account.
Stock Splits and Corporate Actions
Looking solely at BMY’s dividend history leads one to think BMY has occasionally cut its dividend. Investors, however, need to account for stock splits and corporate actions.
Share Repurchases
In May 2010, BMY’s Board of Directors authorized the repurchase of up to $3B of common stock with an additional $3B increase in June 2012. The Board of Directors approved a new share repurchase program authorizing the repurchase of an additional $3B of common stock in October 2016 and in November 2019 this authorization was further increased by ~$7B. In February 2020, the Board of Directors approved an increase of $5B to the total outstanding share repurchase authorization.
The remaining share repurchase capacity under the program was approximately $4.4B as of December 31, 2020, and in January 2021, the Board of Directors approved an increase of $2B to the share repurchase authorization.
The plan is to buy back between $3B – $4B in shares in FY2021; BMY has already repurchased $1.8B in Q1.
Bristol-Myers Squibb – Stock Analysis – Valuation
BMY’s FY2021 adjusted diluted EPS guidance is $7.35 – $7.55. Using the $7.45 mid-point and the current $66.50 share price, the forward adjusted diluted PE is ~8.93.
Forward adjusted diluted EPS guidance from 18 brokers is:
- FY2021 – a mean of $7.47 and a $7.38 – $7.65 range. The forward adjusted diluted PE is ~8.9 using the mean value.
- FY2022 – a mean of $8.04 and a $7.40 – $8.47 range. The forward adjusted diluted PE is ~8.3 using the mean value.
The current BMY is very different from the BMY before the Celgene and MyoKardia acquisitions. As a result, I am not comparing BMY’s current valuation to historical levels.
I think there is a reasonable probability BMY’s valuation will expand. It does not seem to be a stretch to imagine that BMY’s forward adjusted diluted PE could increase to 11 by the end of FY2022. If this occurs, BMY’s share price could very realistically reach ~$88 ($8.04 x 11) thus generating capital appreciation of ~21%.
Bristol-Myers Squibb – Stock Analysis – Final Thoughts
BMY’s attractive valuation presents investors with an opportunity to generate a reasonable rate of return over the next couple of years. However, the $6.4B legal claim is enough to dissuade me from taking a position. Furthermore, I am long MRK and JNJ and view them as superior companies and lean toward increasing my exposure.
Stay safe. Stay focused.
I wish you much success on your journey to financial freedom!
Note: Please send any feedback, corrections, or questions to finfreejourney@gmail.com.
Disclosure: I am long MRK and JNJ. I do not hold a position in BMY and do not intend to initiate one within the next 72 hours.
Disclaimer: I do not know your individual circumstances and do not provide individualized advice or recommendations. I encourage you to make investment decisions by conducting your own research and due diligence. Consult your financial advisor about your specific situation.